The role of chronic inflammation in degenerative diseases associated with aging is considered to be a primary vector for the progression of neurodegenerative disorders and a powerful factor that underlies their etiology.
One needs only to look at the leading causes of mortality—heart disease and stroke, and the research models of inflammation that clearly link the pathogenesis of these disease processes in aging individuals to understand that inflammation and chronic degenerative disease are inseparable.
Since inflammation is central to aging-associated disease processes, it has been heavily investigated in models of neurodegeneration. In Alzheimer’s disease, several studies have sought to clarify whether inflammation is a causative stimulus, or a concomitant feature of the disease process.
Regardless of the etiological focus, the role of chronic inflammation in Alzheimer’s is a well established and the continuing illumination of that knowledge base is vital to the emerging prevention centered paradigm that is growing rapidly.
Pro-inflammatory molecules—Messengers of Inflammation
Several research models have sought to establish the association between quantifiable levels of inflammation in the body and the increased risk it confers in the development of late-onset Alzheimer’s disease (LOAD).
Other models looking to define the pro-inflammatory processes in the Alzheimer’s brain have sought to examine how inflammation arises and perpetuates the characteristic pathology of Alzheimer’s.
Since genetics rule the propensity for the onset of LOAD—numerous other associated risk factors notwithstanding, the identification of “pro-inflammatory” genes in Alzheimer’s have also been heavily studied and identified.(1,2)
In addition, the ApoE4 variant, a genetic risk factor for cardiovascular disease and LOAD, is associated with a pronounced pro-inflammatory profile, while the ApoE3 variant is protective.(3)
Many studies have established that measurable biological markers (biomarkers)* of inflammation is strongly associated with an increased risk for LOAD.
In a subset of the larger Framingham Heart Study, that included 691 healthy people with an average age of 79, circulating cytokines (pro-inflammatory molecules), were tracked for seven years.
The individuals with highest levels of cytokines incurred more than double the risk for Alzheimer’s disease, as those with the lowest amount of cytokines.(4)
3D medical animation still of Cytokines that are important in cell signaling.
Attribution: www.scientificanimations.com—CC BY-SA 4.0
http://www.scientificanimations.com/wiki-images/
Similarly, in a study of 300 subjects with with mild to severe Alzheimer’s, participants that had high baseline levels of the pro-inflammatory cytokine tumor necrosis factor αlpha (TNF-α) were associated with a fourfold increase in the rate of cognitive decline.
Blood levels of TNF-α were measured at two, four and six months. In approximately half of all subjects, increases in the serum levels of TNF-α during the study, were linked to twofold increase in the rate of cognitive decline over a six month period.
Those that had low serum levels of TNF-α did demonstrate any cognitive decline during the six month study timeline.(5)
These examples of pro-inflammatory pathways that elevate the risk for LOAD and its progression demonstrates how aberrant physiological processes related to inflammation can profoundly impact the brain.
So which are the initial drivers of these pro-inflammatory messengers and pathways that spread inflammation in the body and brain?
A major provenance of inflammation patterns that are primary to the disease process inherent in obesity, heart disease, and type 2 diabetes have been established as principal mechanisms that underlie neuroinflammatory pathways in AD.(6)
Please read “Lose The Belly Fat And Save Your Brain From Shrinking”, for a deeper dive into the role of obesity and inflammation in LOAD.
My book, The Diabetic Brain in Alzheimer’s Disease, provides an extensive overview on the link of the aforementioned age-related disease processes to the risk of LOAD and the underlying phenomena of chronic inflammation and oxidative stress that ultimately binds it all together.
Once the cascade of systemic inflammation permeates the central nervous system (CNS), it activates immune cells (microglia) that ramp up pro-inflammatory pathways, which in turn can trigger the seminal pathological events that characterize the early stages of Alzheimer’s.
Left unrestrained, these neuroinflammatory response in the CNS has been shown to precede the prototypical lesions—plague and neurofribrillary tangles (NFTs), and the subsequent progression to Alzheimer’s dementia.
Indeed, the phenomena of neuroinflammation is a core feature that characterizes the earliest and the more advanced neuropathological features of Alzheimer’s.(6)
Next, I will describe some pivotal points that highlight the role of the microglia in chronic inflammation and Alzheimer’s.
The Brain Microglia & Inflammation
In the CNS, a collection of specialized cells referred to as the microglia cells,** are the resident immune cells of the CNS.
Microglia perform immunoprotective and essential CNS maintenance functions, including the removal of debris and pathogens, and the “pruning” of synapses. The latter is an important sculpting process (remodeling) that ‘weeds” out neural connections in a developing brain.(8,9)
Ultimately, the role of microglia in surveying, protecting, and remodeling the neurological terrain is vital in a healthy CNS are part and parcel of their activation.
However, excessive and chronic microglial stimulation may eventually result in an overactivation state that provokes the pro-inflammatory profile and toxic cascade that epitomizes Alzheimer’s neuroinflammation.
Astrocytes are activated in neuroinflammatory responses as well. See image below.
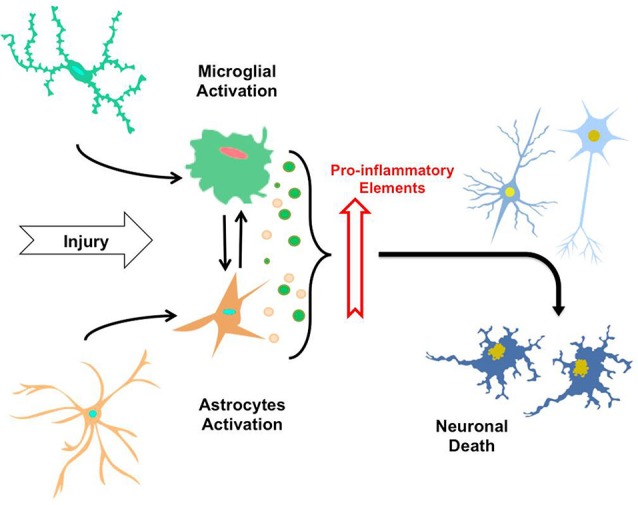
“The neuroinflammatory process. By sensing signals of damage or injury, astrocytes and microglia suffer a gradual activation process, leading to morphological changes and secretion of pro-inflammatory elements (i.e., cytokines, cytotoxic elements, ROS). Thus, the constant exposure of astrocytes and microglia to factor causing injuries and secretion of these elements induce mutual activation of microglial cells and astrocytes, along with neuroinflammatory process that eventually trigger neuronal death.”
Source and attribution: CC By 3.0
Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. https://www.researchgate.net/publication/262055532_Neuroinflammation_in_the_pathogenesis_of_Alzheimer’s_disease_A_rational_framework_for_the_search_of_novel_therapeutic_approaches
In dementia disorders associated with Alzheimer’s or vascular disease, typical inflammation driven insults in the central nervous system include brain trauma (TIAs/stroke), and aberrant beta-amyloid and tau protein aggregation. (see article: Plaques and Tangles in the Alzheimer’s Brain-Which One Is Most To Blame For Alzheimer’s Disease?)
Microglial activation is an essential component in the repair process following trauma, and in the attempt to contain proliferation of aggregates of beta-amyloid and tau protein—plaques and tangles, via phagocytosis (engulfing and degradation of pathogens and cell-derived debris).
Beta-amyloid and tau protein aggregates are a primary stimulus in the activation of microglia, and this pathogenic cascade begins early in the time spectrum of Alzheimer’s disease.(10)
However, if these initial triggers of microglial activation are not sufficiently attenuated and buffered by innate anti-inflammatory and antioxidant reserves or therapies, a chronic neuroinflammatory cascade may ensue that induces an overactivation of the microglia.(1)
Additionally, there is also bountiful evidence to assert that various inflammation pathways and factors that arise from outside the brain are a significant component in the genesis associated with an increased risk for LOAD.
The pro-inflammatory vicious cycle may originate from:
- cardiometabolic disease (type 2 diabetes and cardiovascular disease),
- hypertension
- chronic infections
- neurotoxic exposures to metals such as mercury and other toxic chemicals,
- a pro-inflammatory diet,
- chronic stress,
and many other epigenetic (environmental triggers) and genetic susceptibility factors that upregulate inflammation in the brain.
Anti-inflammatory Drug Interventions
To date, there is not medical silver bullet that delivers any effective long term therapeutic intervention for Alzheimer’s disease (AD).
The current FDA-approved drugs do not provide any significant disease-modifying benefits, and anti-inflammatory drug trials and interventions have yielded less than stellar results.
A few epidemiological studies*** have shown a reduced risk for AD in individuals taking nonsteroidal anti-inflammatory drugs (NSAIDs).****
Juxtaposed to these findings are trials that revealed no benefits in reducing cognitive decline in patients with AD.
The contrasted outcomes highlights the growing consensus that anti-inflammatory interventions are more wisely applied in the early stages of AD.(3)
Are NSAIDs a viable solution to prevention of AD?
The modulation of inflammation is central to the reduction of risk associated with the onset of AD, and for the management of the disease process.
However, prolonged use of NSAIDs is not advisable or recommended.
Long term NSAID therapy is associated with severe side effects (SEs).
SEs from chronic NSAID use include gastrointestinal damage, heart failure, liver and kidney damage.
The alternative anti-inflammatory solution?
As before, microglial activation, and the role of related pro-inflammatory pathways associated with the pathomechanisms***** of cognitive decline, and the progression to LOAD or vascular dementia, begins in the early stages of the disease process.
Once the damage is evidenced by the hallmark plaques and tangles that represent an advanced disease pattern evidenced in Alzheimer’s disease, there may be little that is clinically achievable, albeit some stabilization and slowing the progression of the disease process may be possible.
Nevertheless, there is promise in early assessment and intervention strategies.
Rather than the disease centered model of pharmacological interventions, the real promise in the potential prevention of AD lies in the tried and true dietary, nutrition (nutraceuticals)******, and lifestyle interventions that are shown to be effective in all of the chronic degenerative diseases associated with inflammation.
Nutrition and Lifestyle
Dietary and nutrition therapies that provide anti-inflammatory and anti-oxidative protection for individuals have consistently demonstrated benefits in reducing the risk for LOAD.(11,12,13,14)
Studies investigating ketogenic diet therapy, caloric restriction, and intermittent fasting are demonstrating remarkable promise in reversing cardiometabolic disease and sharply mitigating the pro-inflammatory and oxidative stress mechanisms that link the latter to LOAD.(15,16,17,18)
The fields of nutritional genomics (nutrigenomics) and nutrigenetic approaches to a personalized diet and nutrition protocol based on an individual’s genetic profile and nutritional requirements provide an additional layer of insight that allows for precision nutrition interventions.
Exercise and stress reductions habits that include meditation have also shown to be protective against chronic inflammation and age-related disorders including LOAD.
Personalized nutrition, diet, and lifestyle interventions can reverse years of damage incurred by unhealthy eating and lifestyle patterns, and set the foundation for a vital body and brain for the rest of your life.
Do you desire a helping hand in your personalized brain health program that incorporates a science-based approach to your unique needs?
My BrainDefend® Personalized Body-Brain Heath and Renewal Program is designed to provide that for you! Schedule a Complimentary Discovery Session and learn how BrainDefend® can change your brain and your life.
Recommended reading on nutrition:
Alpha lipoic acid Protects Brain Cells–Antioxidant Mechanisms For Alzheimer’s Prevention
Polyphenols in the Protection Against Cardiovascular Disease and Dementia
Endnotes
* Biomarker—A measurable substance, biochemical, genetic, or molecular, in an organism that may be indicative of some disease state, or the risk for it.
** Microglia—Microglial cells are one of the three main types of central nervous system glia. The central nervous system (CNS) consists of neurons and glial cells. The three types of CNS Glial cells are Astrocytes, Oligodendrocytes, and Microglia. Glial are commonly described as the “supporting cells” of the nervous system.
*** Epidemiology: In medical science, epidemiological studies investigate the patterns and causes, and control of disease in human populations
**** NSAIDs (Nonsteroidal anti-inflammatory drugs) are a classification of prescription, and over-the-counter (OTCs) medications for the treatment of pain and inflammation related disorders. NSAIDs function by inhibiting both cyclooxygenase 1 and 2 (Cox-1 and Cox-2) enzyme pathways that form pro-inflammatory signaling molecules (prostaglandins). Examples of NSAIDs include: Ibuprofen, naproxen, Celebrex and aspirin.
***** Pathomechanism refers to the pathological mechanism by which a pathological condition associated with a disease occurs. The prefix patho-, is derived from the Greek “pathos” meaning “suffering or disease.”
******Nutraceuticals: The term nutraceutical was first coined in the 1990’s by Dr. Stephen DeFelice, and was defined as: “A nutraceutical is any substance that is a food or a part of a food and provides medical or health benefits, including the prevention and treatment of disease”. Currently, the term is often used to describe a dietary supplement, Medical Food, or food product that provides health and medical benefits, including the prevention and treatment of disease.*
References:
1. Pro-inflammatory gene expression and neurotoxic effects of activated microglia are attenuated by absence of CCAAT/enhancer binding protein β
Marco Straccia, Núria Gresa-Arribas, Guido Dentesano, Aroa Ejarque-Ortiz, Josep M Tusell, Joan Serratosa, Carme Solà, and Josep Saura
Journal of Neuroinflammation 2011, 8:156
2. Polymorphisms in Inflammatory Genes and the Risk of Alzheimer Disease
Patrick L. McGeer, MD, PhD; Edith G. McGeer, PhD
Arch Neurol. 2001;58(11):1790-1792
3. Neuroinflammation in Alzheimer’s disease wanes with age.
Hoozemans JJ1, Rozemuller AJ, van Haastert ES, Eikelenboom P, van Gool WA.
J Neuroinflammation. 2011 Dec 7;8:171.
4. “Inflammatory markers and the risk of Alzheimer disease: The Framingham Study.”
Tan ZS et al.
Neurology 2007;68:1902-1908.
5. Systemic inflammation and disease progression in Alzheimer disease
C. Holmes, C. Cunningham, E. Zotova, J. Woolford, C. Dean, S. Kerr, D. Culliford, and V. H. Perry
Neurology September 8, 2009 vol. 73 no. 10 768-774
6. LINKING CARDIOMETABOLIC DISORDERS TO SPORADIC AD: A PERSPECTIVE ON POTENTIAL MECHANISMS AND MEDIATORS
Narayan R. Bhat
J Neurochem. Nov 2010; 115(3): 551–562.
7. Neuroinflammation – an early event in both the history and pathogenesis of Alzheimer’s disease.
Eikelenboom P1, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, van Gool WA.
Neurodegener Dis. 2010;7(1-3):38-41.
8. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development
Rosa C. Paolicelli, Giulia Bolasco, Francesca Pagani, et al.
Science 9 September 2011: Vol. 333 no. 6048 pp. 1391-1392
9. Microglia and synapse interactions: fine tuning neural circuits and candidate molecules.
Miyamoto A1, Wake H, Moorhouse AJ, Nabekura J.
Front Cell Neurosci. 2013 May 15;7:70.
10. The significance of neuroinflammation in understanding Alzheimer’s disease.
Eikelenboom P1, Veerhuis R, Scheper W, Rozemuller AJ, van Gool WA, Hoozemans JJ.
J Neural Transm. 2006 Nov;113(11):1685-95. Epub 2006 Oct 13.
11. Dietary Modulation of Oxidative Stress in Alzheimer’s Disease.
Thapa A, Carroll NJ.
Int J Mol Sci. 2017;18(7):1583. Published 2017 Jul 21.
12. The Role of Selected Bioactive Compounds in the Prevention of Alzheimer’s Disease
Wojciech Grodzicki and Katarzyna Dziendzikowska
Antioxidants 2020, 9(3), 229
13. Nutrition and prevention of Alzheimer’s dementia
Arun Swaminathan and Gregory A. Jicha
Front. Aging Neurosci., 20 October 2014
14. Nutrition for the ageing brain: Towards evidence for an optimal diet
D. Vauzour et al.
Ageing Research Reviews 35 (2017) 222–240
15. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies.
Maalouf M, Rho JM, Mattson MP.
Brain Res Rev. 2009;59(2):293–315.
16. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease
Alessandro Pinto, Alessio Bonucci,Elisa Maggi,Mariangela Corsi and Rita Businaro
Antioxidants 2018, 7(5), 63
17. Effects of Intermittent Fasting on Health, Aging, and Disease
Rafael de Cabo, Ph.D., and Mark P. Mattson, Ph.D.
N Engl J Med 2019; 381:2541-2551
18. Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer’s disease-induced estrogen deficient rats.
Shin BK, Kang S, Kim DS, Park S.
Exp Biol Med (Maywood). 2018;243(4):334–343.
* These statements have not been evaluated by the Food and Drug Administration.





